Abstract
Despite many ongoing clinical drug trials and advances in acute myeloid leukemia (AML) therapy, the 3+7 induction regimen with anthracyclines and cytarabine (+/- midostaurin) remains the golden standard of AML treatment. Approximately 40 % of AML patients do not achieve a complete hematological remission (CR) after the first cycle of 3+7 chemotherapy and face an adverse prognosis. One of the main causes of treatment failure is chemoresistance of leukemia cells acquired by various mechanisms, all of which have not been identified as yet. We present here a unique study of transcriptome landscape mapping of separated AML blasts from paired samples collected before and after chemotherapy administration, focusing on differences between responding (RES) and refractory (REF) AML.
The aim of this study was to describe transcriptional changes in leukemic blasts between RES and REF AML patients after chemotherapy administration, and to identify molecular pathways involved in reduced sensitivity to chemotherapy.
Leukemic blasts were sorted from peripheral blood of AML patients collected at diagnosis (day 0, n=30) and on the third day (day 3, n=16) of 3+7 chemotherapy. CD34+ and CD117+ antibodies were used for blast separation on autoMACS (Miltenyi Biotec). 100 ng of high quality DNAse-treated RNA (RIN>8) was ribodepleted via the RiboCop rRNA Depletion kit (Lexogen) and the library was prepared using the NEBNext Ultra II Directional RNA Library Prep Kit (NEB). Transcriptome was sequenced on NovaSeq (Illumina). For data analysis, we divided patients by primary response status into RES and REF groups based on CR achievement after the first cycle of 3+7 therapy. Quantification of gene expression was performed using StringTie2 software (version 1.3.6). Resulting data were subsequently analyzed and visualized in R software 4.0.0 using edgeR and pheatmap packages. The String and GO databases were used to annotate genes into molecular pathways and biological processes.
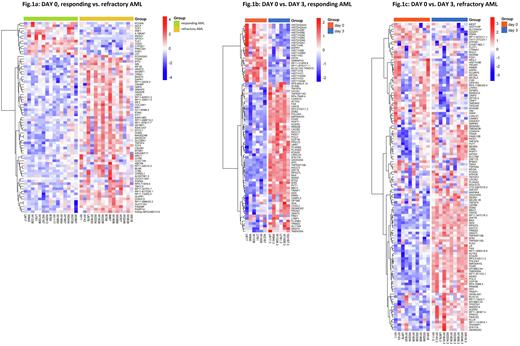
We identified 75 differentially expressed genes (FDR<0.05) at day 0 between RES (n=15) and REF (n=15) AML patients (Fig.1a). Among genes upregulated (n=62) in REF AML, those previously associated with inferior prognosis such as MN1 (transcriptional regulator), ABCA6 (membrane transporter) and SETBP1 (transcriptional regulator) were identified. On the contrary, PRF1 (involved in killing transformed cells) and TLE1 (negative regulator of NF-κB) were among genes downregulated (n=13) in REF AML. In general, genes upregulated in REF AML were assigned to Golgi membrane, carbohydrate transport and interferon signalling and genes upregulated in RES AML to immune response and the β-catenin/TCF transcriptional complex. In the pairwise analysis (day 0 vs. day 3), we identified 82 differentially expressed genes in RES (Fig.1b, n=6) and 121 differentially expressed genes in REF AML (Fig.1c, n=10). There were 42 genes identically deregulated in both groups and they were annotated into DNA damage/stress response, DNA repair and apoptotic pathways. Next, we compared genes that were deregulated only in RES or REF AML. In RES AML, there was a big cluster of downregulated histone and chromatin remodelling genes that was missing in REF AML. Upregulated genes in RES AML included those with antiproliferative, proapoptotic and p53 stabilizing functions. In REF AML, the principal deregulated genes were those affecting cytoskeleton remodelling (downregulated), p53 signalling network (upregulated) and genotoxicity pathways (upregulated).
We identified differences in leukemic blast transcriptome between RES and REF AML before and after chemotherapy administration. The role of some differentially expressed genes has already been elucidated in AML pathogenesis, others deserve a closer look to evaluate their relevance as potential targets for emerging therapies. Instant downregulation of histone genes after chemotherapy administration seems to be a strong sign of RES AML and requires further attention for its use as a biomarker for early stratification into good versus poor responder AML.
This project was supported by Ministry of Health of the Czech Republic (00023736, IHBT) and by GA CR (No. 20-19162S).
Disclosures
Salek:Amgen: Consultancy, Research Funding; Incyte: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal